Matter is defined as anything that has mass and volume (occupies space). Note that the term ‘mass’ is used to define, not “weight”. This is because mass of matter is the same in everywhere, while weight can change depending on the gravity. Mass and volume are properties of matter. For example, stone and air are both matters since they have mass and volume. As for air, its mass and volume are hard to observe because it takes the form of gas. So, in order to study matter, we have to know its properties and its state/phase well.
Matter is defined as anything that has mass and volume (occupies space). Note that the term ‘mass’ is used to define, not “weight”. This is because mass of matter is the same in everywhere, while weight can change depending on the gravity. Mass and volume are properties of matter. For example, stone and air are both matters since they have mass and volume. As for air, its mass and volume are hard to observe because it takes the form of gas. So, in order to study matter, we have to know its properties and its state/phase well.
Discussion on matter in this section will cover its properties, states, and changes. Besides that, the classifications of matter will also be discussed.
1. Properties of Matter
The properties of matter can be classified based on the amount of matter and on the changes that matter undergoes.
» Based on the amount of matter, the properties of matter are classified into intensive property and extensive property.
» Based on the changes that matter undergoes, the characteristics of matter
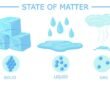
2. States of Matter
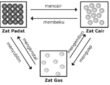
There are 3 states of matter: solid, liguid, and gas. Consider rock, water, and air. If a rock is placed inside a glass, its shape does not change. However, when you put water inside the glass, its shape changes to the shape of the glass. And of course an empty glass is actually filled with air which is easy to move when a rock or water is filled in the glass.
To understand the differences among solid, liguid, and gas, we can use the models below. Matter is considered to be composed of round particles, and the differences among the states are related to the distance between particles and the movement of the particles within the matter.
Matter can change from one state to another. For example, when ice is heated, it can change into water and vapour. The same thing goes for vapour that changes into water and ice when it is cooled. This type of change is called change of state (change of phase). Note that in change of state, the type of matter does not change (ice, water, and steam are the same matter).
Change of state occurs due to energy change, such as by heating or cooling. Change of energy affects the attracting forces between particles composing the matter. The bigger the attracting force between particles, the closer the distance between particles; and vise versa.
3, Changes in Matter
Changes in matter can be classified into:
a. Physical change, defined as a change that does not produce new matter. This change only involves a change in shape or state of matter. For example, the change of ice to water and dissolving sugar in water. Physical change can be easily reversed into its original state.
b. Chemical change or chemical reaction, defined as a change that produces new matter. For example, burning wood to ashes, iron rusting to iron oxide, and reaction between sodium metal and chlorine gas to sodium chlorine (salt). A chemical change is difficult to be reversed to its original state.
To find out whether a chemical change/ reaction takes place, there are several observations that can be made, such as change in color, formation of gas, formation of precipitate, and change in temperature. Physical change and chemical change/ reaction are often encountered in industrial process. For instance: Separating gasoline from petroleum involves physical change because gasoline is amongst the various materials composing petroleum. The process of creating aluminum from bauxite ores involves chemical change because aluminum is a new matter that previously did not exust.
4. Classification of Matter
To help learn about the millions of matter we have in the world, chemists have classified matter as follows.
a. Elements
Elements are substances that cannot be decomposed into simpler substances by chemical reactions. For example, sodium chloride (table salt) can be decomposed into sodium metal and chlorine gas. Sodium and chlorine gas cannot be decomposed into any simpler substances through chemical reaction. Thus, sodium and chlorine are elements. Scientists have discovered 90 elements and synthesized 25 more, thus resulting in a total of 115 elements.
For practical reasons, elements can be written using letters as symbols, proposed by Swedish scientist Jons Jakob Berzelius (1799-1818). The symbol is stated in a capital letter deriving from the Latin name of the element. For example, H for hydrogen (hydrogenium), O for oxygen (Oxygenium), and C for carbon (Carbonium). If there are other elements that begin with the same word, then it is necessary to add a second letter written in lowercase. For example, Ca for calcium (Calcium) and Cd for cadmium (Cadmium) since C is already used for carbon (Carbonium).
b. Compound
Compound is a combination of two or more elements into a complex substance. For example, sodium and chlorine can react to form a compound of sodium chloride (salt).
Sodium + Chlorine —> Sodium chloride
A compound has different properties from its composing elements. For example, a sodium chloride (compound) has different properties from sodium and chlorine.
c. Mixture
Mixture is a combination of two or more pure substances (elements,compounds) without involving a chemical reaction. Seawater, air, and syrup are examples of mixtures. Seawater is a mixture of water, sodium chloride and other compoungds, air is a mixture of nitrogen, oxygen, carbon dioxide and other gases, and syrup is a mixture of water and sugar. Mixtures can be grouped into solution, colloid, and suspension. The differences between these groups are related to the sizes of their particles. Properties of pure substances are retained in a mixture. For example, the sweet taste from sugar can still be found in syrup.